Activity Description/Rationale
This is a series of lessons that discusses the types of water pollution the students are testing for more in depth, and ends with an overview type of lesson on water pollution.
NYS/CCL Standards (Content Knowledge, IAD)
RST.11.12.2
WHST.9.12.2
RST.11.12.8
Goals: Process Skills (Basic & Integrated) and Attitudes/ (Enduring Understandings & Essential Questions)
Students will learn how to think critically about water quality and the tests they have performed. They will learn what it means to interpret the results of a test, and how this will impact their water quality lab reports.
Universal Design for Learning/Differentiation
These are real tests that water managers perform, and students will work as a class to fully interpret them, and gain a more complex understanding of water and its potential contaminants.
Materials
Water quality tests (optional)
Estimated Length of Activity:
5 class periods (3 hours 45 mins – 4 hours 10 mins)
Pre-Activity
Discussion of the water cycle would be beneficial.
Activity Instructions:
Lesson 1: Water pollution overview
Go over powerpoint lecture with students. It may be a bit wordy, so be sure to print out the lecture prior to teaching so students don’t struggle to copy everything down. Take your time, but use this to introduce the types of general contamination that affects water bodies. This will be relevant in the following week when you discuss water contamination and interpretation of the class’s results.
Lesson 2: pH and Water Temperature
(5-10 minutes) have students write down how pH and/or temperature can affect water quality. Discuss the responses as a class. The following lesson should advance their knowledge past what you’ve previously discussed.
Remainder of class: pH is the number of hydrogen ions. The scale is a logarithmic scale, ask students how this may influence the values. Discuss that freshwater generally ranges from 5.5-6.5. Why isn’t freshwater 7? What affects it to make it more acidic? Discuss that snow and rainwater may be lower. Why would they be even lower? Bring up the topic of acid rain/precipitation. They may have seen old graves in a cemetery which are difficult to read. This is partially due to acid precipitation. How would you interpret the results of the tests the class did based on pH? What conditions at the site or in the watershed might have led to this pH level? What types of fish and/or plants can and cannot survive at this pH?
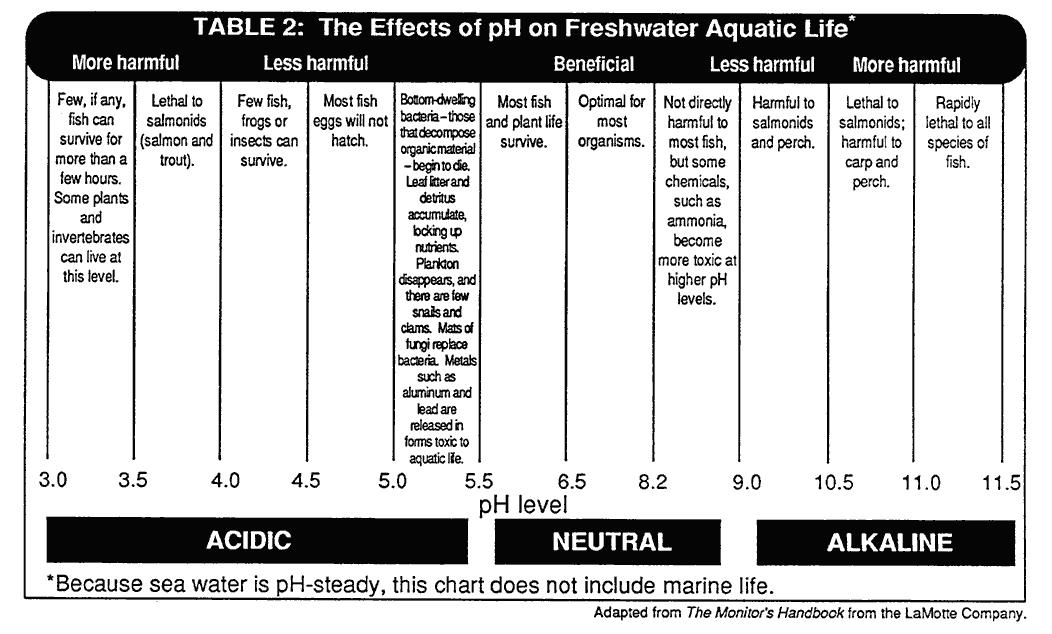
If you have extra time, you may want to test household items, such as soda, baking soda, asprin, tobacco sauce, etc.
Temperature should have been measured in the field. Make sure students understand why this is important. Should discuss factors affecting water temperature, and the effects of raising the water temperature, especially for organisms that live in the water. Discuss the data collected by the class. What factors at the site contributed to the water’s temperature? How large was the water body? Was the water still or moving? Was there vegetation? Did it provide shade? What season did you take the samples? What time of day? How about sources of thermal pollution?
End with the discussion of how water temperature affects other water quality factors, such as DO levels are higher in colder water, higher temperatures speed up chemical reactions in plants and animals (and metabolisms).
Fish temperature ranges: Native brook trout – 55F in summer
Rainbow trout and salmon – 55F-68F in summer
Carp and catfish above 68F in summer
Plant life temperature range: Green algae bloom above 77F
Blue green algae above 86F
Many fresh water aquatic plants above 68F, few below 55F
Lesson 3: DO and BOD
(5-10 minutes) have students write down how DO and/or BOD can affect water quality. Discuss the responses as a class. The following lesson should advance their knowledge past what you’ve previously discussed.
Remainder of class: DO is a measure of the dissolved oxygen contained in the water. It enters through mixing with the atmosphere, and though photosynthesis by plants. To interpret the results, think about these standards: 
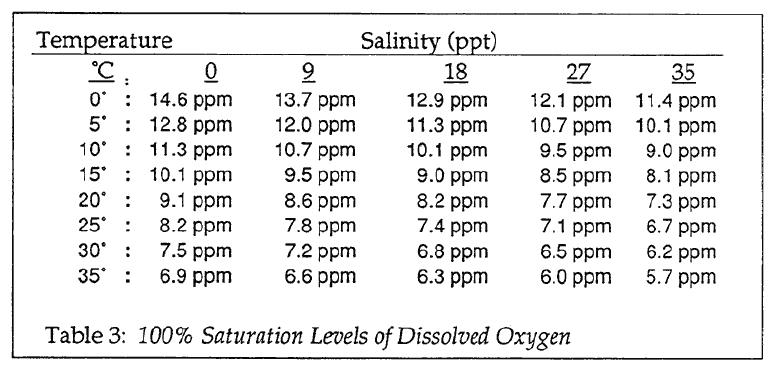
In the interpretation look at table 3. The results should be close to these numbers for healty water. Does the water have enough oxygen to support a healthy fish population? What factors may affect the amount of DO? Was the water choppy or near a dam? Was the water warmer or cooler? Was there lots of vegetation in the water? Was it decaying or alive?
The BOD test is similar, although it takes place after 5 days of incubation. Why is it necessary to wait 5 days? BOD results indicate amount of organic material in the water sample. The more organic material, the more bacteria will consume oxygen to decompose that organic material. An unusually high BOD level may indicate the presence of pollution or sewage. 
Have class interpret results and determine factors that may have affected these levels.
Lesson 4: TDS and Salinity/Turbidity
(5-10 minutes) have students write down how TDS and/or Salinity/Turbidity can affect water quality. Discuss the responses as a class. The following lesson should advance their knowledge past what you’ve previously discussed.
Remainder of class: The test for TDS is based on electrical conductivity in the water. Some solids are essential to maintaining health, others deter from the health of the water body. Interpreting the class results will show the how many salts and minerals are dissolved in the water. Think about what may contribute to the TDS of the water. Were there lots of plants in the area? What kind of soil is the water on? Is the water hard or soft? Use the table to interpret results.

Turbidity, which seems like TDS, is a measure of how much light passes through the water, and is caused by the suspended solid particles. More turbid water looks ‘grosser’ but it may be healthier than clear water. Why might this be? How do students interpret the results? Is the amount of turbidity a sign of healthy or unhealthy water system? What might account for the level of turbidity? Weather can play a role, as can seasons? Think about the time of year the sample occurred. Also algae blooms can cause higher turbidity. Contamination from pollutants like industrial waste, urban runoff, or sewage also are contributing factors.
Lesson 5: Total coliform bacteria and Nitrates
(5-10 minutes) have students write down how total coliform bacteria and/or nitrates can affect water quality. Discuss the responses as a class. The following lesson should advance their knowledge past what you’ve previously discussed.
Remainder of class: Total coliform bacteria is only an indicator. So, if the test results are positive, is it definitive proof of water contamination? The class results should be interpreted. Positive results indicate the presence of total coliform bacteria from warm-blooded animals and various soil organisms. People shouldn’t drink the water! Why not? What might the source be? Did you see signs of animals nearby? Did you see outflow pipes or septic tanks that might contribute to the bacteria levels?
Nitrates can act as a fertilizer for plant life. Why might it be bad to fertilize the aquatic plants? What would happen to the water? Children have even been poisoned by drinking formula in water with high nitrate levels (blue baby syndrome). The test can be interpreted by looking at the levels of nitrate in the sample. Nitrate levels around 10 ppm are unsafe for drinking. It’s hard to determine the precise level of nitrates at which problems begin to occur because there are so many factors involved (as with all our tests). If there were a low pH, it wouldn’t matter how high the nitrates were, because little life would grow. If there were warm conditions with adequate oxygen, a little nitrate would cause a significant change in the ecosystem. The movement of the water also plays a role, because faster moving water negates the effect of the nutrients. The following are general standards from NOAA.
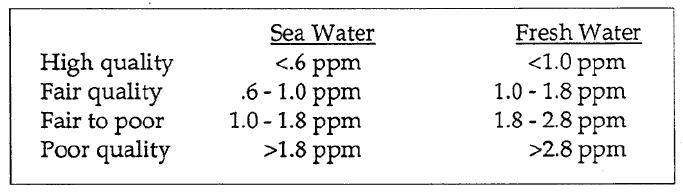
Assignments
There are no specific assignments. You may wish to have students interpret the results of the class tests and use them as grades, or have students continue working on their lab reports.
Assessment and Reflection
Assessment is based on their interpretation of the results. If students are struggling with interpreting the types of responses the got from the field trip water samples go over the material again. It’s important they understand these tests for their lab reports.
Instructor’s Notes:
This set of lessons is to help students better interpret their results for the lab report. If it makes sense, choose to discuss only the relevant tests to your particular unit.
